Megha Thakur 2025-12-10
In manufacturing, most problems don’t start on the shop floor. They start much earlier, with materials that don’t behave the way they should.
A batch cures too fast, another takes longer than expected. Finished products look fine today but fail sooner than they should. Often, the machinery gets blamed but very often, the real reason is far simpler: the raw material wasn’t consistent.
Zinc Oxide is one such material. It’s used across industries, especially rubber, and it plays a far bigger role in manufacturing stability than it gets credit for. When chosen and handled correctly, it reduces risk.
What exactly is Zinc Oxide?
Zinc Oxide, or ZnO, is an inorganic compound made of zinc and oxygen. In most industrial forms, it appears as a fine white powder. It doesn’t dissolve in water, remains stable at very high temperatures, and reacts in controlled ways with other chemicals.
These traits make it extremely useful in manufacturing. ZnO doesn’t break down easily under heat, it doesn’t lose its structure during processing, and it behaves predictably when combined with other materials. That predictability is the reason it’s trusted in processes where even small variations can lead to losses.
Zinc Oxide is not used because it is trendy or new. It is used because it works consistently.
Its most important role is in rubber manufacturing, where it acts as an activator in sulfur vulcanization. Simply put, ZnO helps the rubber cure properly. It supports the chemical reaction that turns soft rubber into a strong, elastic, usable product.
Without Zinc Oxide, vulcanization becomes inefficient and unpredictable: cure times fluctuate, mechanical strength drops, heat resistance weakens. This is why tyres, seals, belts, hoses and footwear soles almost always rely on ZnO as a core ingredient.
Beyond rubber, Zinc Oxide is used in:
• Ceramics and glass, to improve thermal stability and surface quality
• Paints and coatings, for UV protection and durability
• Concrete and construction materials, for moisture resistance
• Electronics, sensors and optoelectronics, due to its semiconductive properties
• Personal care and pharmaceuticals, for its soothing and protective nature
Its wide range of uses comes down to one thing. It performs reliably under stress.
From a manufacturing point of view, Zinc Oxide is valuable because of how it behaves during processing, not because of how it looks on paper.
It remains stable at high temperatures, which means it doesn’t degrade during curing or firing processes. It absorbs ultraviolet radiation, helping finished products last longer in outdoor conditions. It reacts efficiently with accelerators and other formulation agents, helping chemical reactions complete properly instead of partially.
Most importantly, when Zinc Oxide quality is consistent, the entire production process becomes easier to control.
Manufacturing risk usually shows up in four places. Zinc Oxide influences all of them.
1. Process inconsistency
If cure times vary from batch to batch, production schedules suffer. Reliable Zinc Oxide helps stabilise vulcanization and reaction rates, making processing more predictable.
2. Product failure after dispatch
Many product failures happen after exposure to heat, pressure or sunlight. ZnO improves resistance to ageing, heat build-up and UV damage. That reduces early failures and warranty claims.
3. Scrap and rework
Unstable formulations increase rejection rates. When Zinc Oxide disperses evenly and reacts as expected, mechanical properties stay within range. That means fewer rejected parts and less rework.
4. Compliance and documentation risks
Well-defined Zinc Oxide specifications and testing records make audits smoother. They also help manufacturers stay aligned with environmental and safety regulations without last-minute corrections.
One common mistake manufacturers make is assuming all Zinc Oxide is interchangeable. It isn’t.
Particle size, purity, surface area and manufacturing method all affect how ZnO behaves in a formulation. Small changes can impact dispersion, reaction speed and final properties.
This is why ZnO needs to be treated as a controlled input, not a commodity powder that can be swapped without testing.
This is also where Rajshila often steps in, helping manufacturers align material characteristics with real process requirements, rather than just chasing availability.
In tyre manufacturing, inconsistent Zinc Oxide can lead to uneven curing, heat build-up during use, and premature wear. In seals and gaskets, poor ZnO dispersion can cause cracking after UV exposure. In coatings, low-quality ZnO reduces protection and shortens repaint cycles.
In each case, the issue isn’t dramatic, it’s gradual and that’s what makes it risky. Problems appear slowly, after products leave the factory.
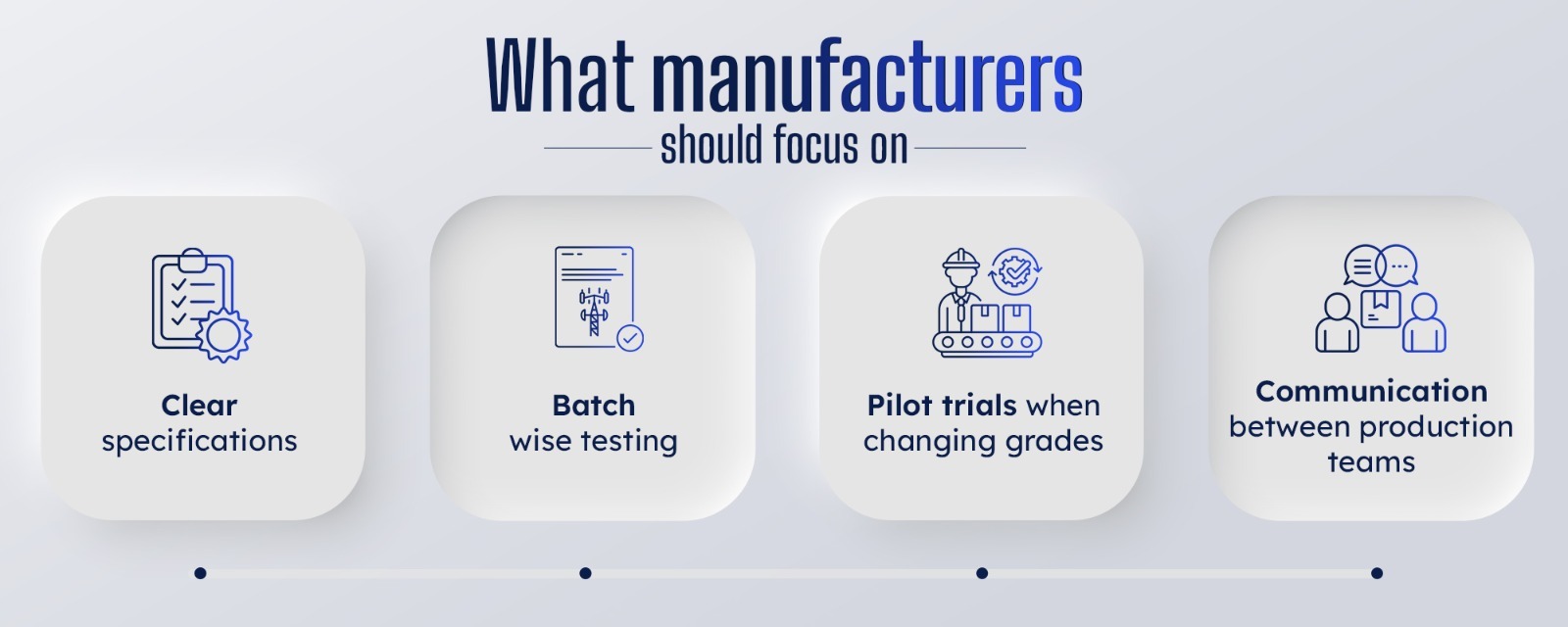
 When these basics are in place, Zinc Oxide becomes what should be a stabilising force, not a variable.
When these basics are in place, Zinc Oxide becomes what should be a stabilising force, not a variable.
Zinc Oxide doesn’t solve manufacturing problems on its own. But when it’s reliable, it removes a layer of uncertainty from production. And in manufacturing, fewer unknowns mean fewer failures.
If you’re looking to tighten control over your formulations, improve process stability, or simply reduce the number of surprises on your production line, it’s worth taking a closer look at how Zinc Oxide fits into your system.
At Rajshila, the focus is simple, helping manufacturers make informed material decisions that protect quality, efficiency and long-term performance. If that’s a conversation you’re ready to have, let’s connect now.